“While the COVID-19 pandemic taught new lessons about the quick and safe delivery of critical vaccines, the global shock is only now being understood.” That’s the opening observation of the 98-page 2022 Life Sciences Report, published by the AC firm CRB and based on responses from nearly 500 industry leaders, which explores this industry sector’s R&D and growth strategies.
This is CRB’s third Horizons report, and its first to include Europe, where many of the industry’s leading organizations are paving the way through innovation, groundbreaking research, and new and dynamic ways of speeding therapies to patients.
CRB is seeing a rapidly maturing industry that’s in pursuit of more diversification. “The days of single-product specialization are receding,” the report states, as companies large and small are utilizing a wide array of tools to expand their pipeline and address diverse indications.
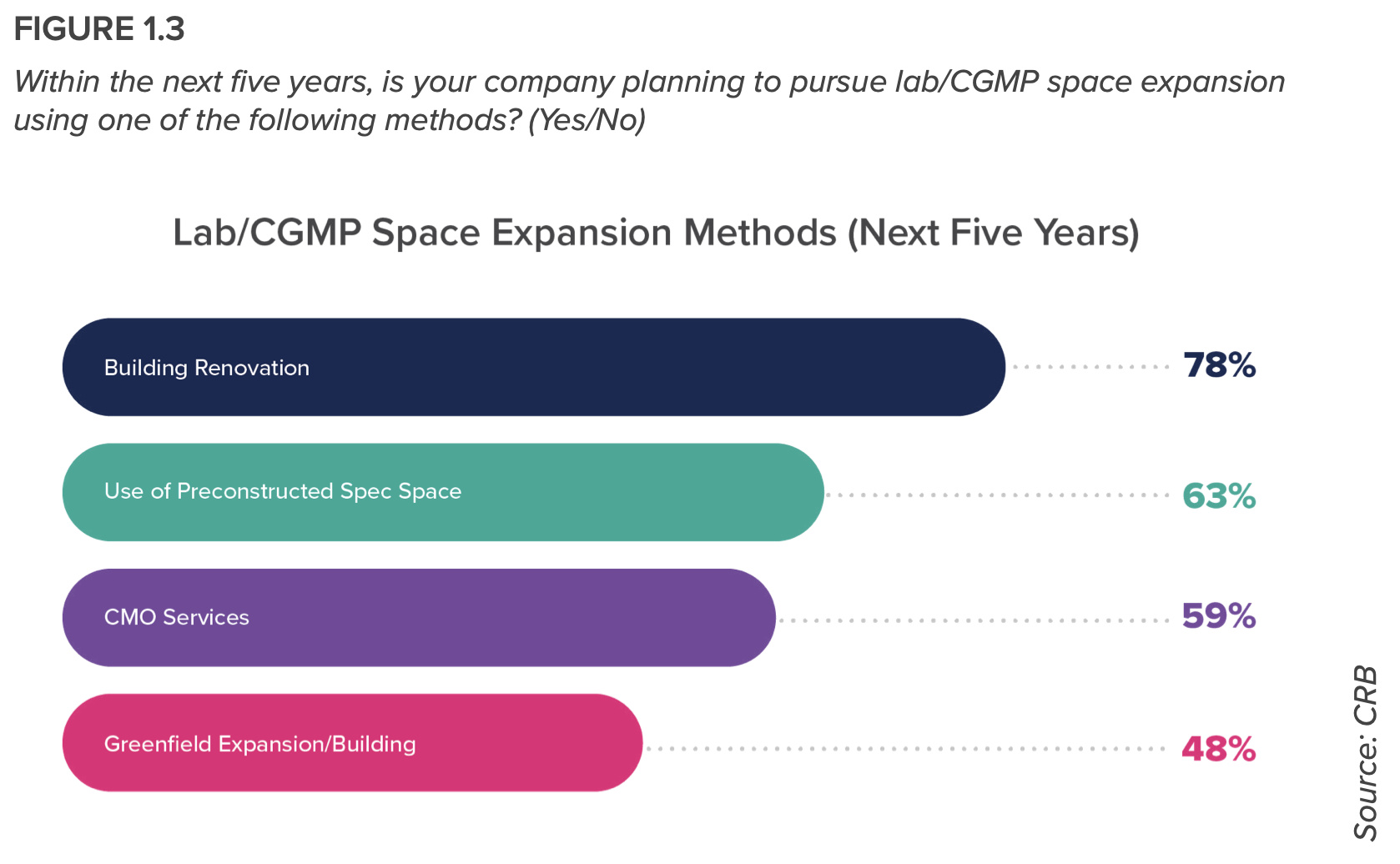
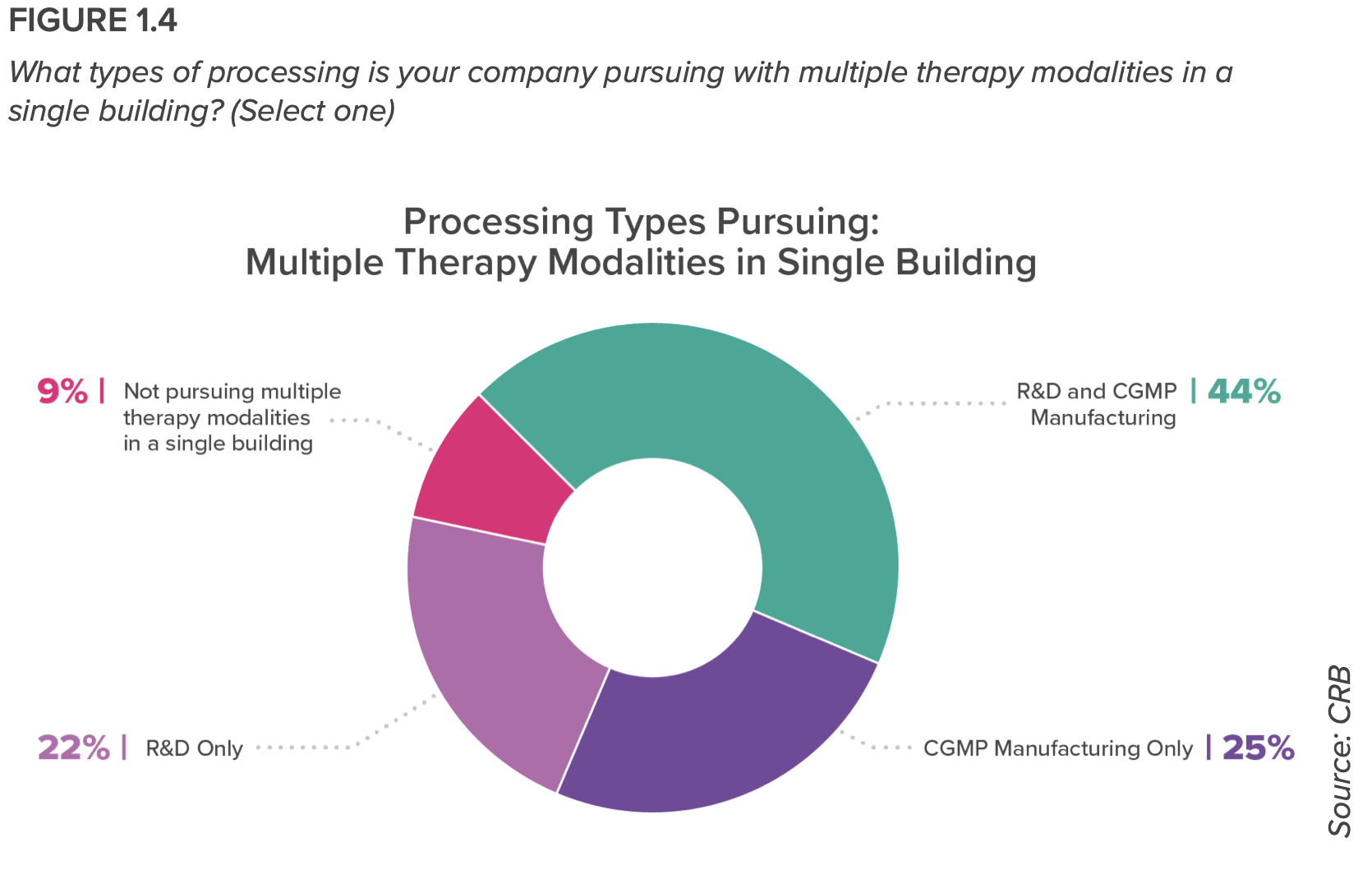
Nearly all respondents (90%) are developing and manufacturing multiple therapy modalities in a single building, or plan to do so in the future. And more than half of the respondents plans to rely on contract development and manufacturing organizations (CDMOs) over the next three years. “We’re seeing a rise in hybrid models—that is, owners who are offering their in-house manufacturing expertise for hire,” the report states.
As such, the nature of in-demand talent is changing, as companies mature towards more automated, AI-driven manufacturing models, with the traditional C-suite expanding to include roles previously unseen in this industry, such as “Chief Data Officer.”
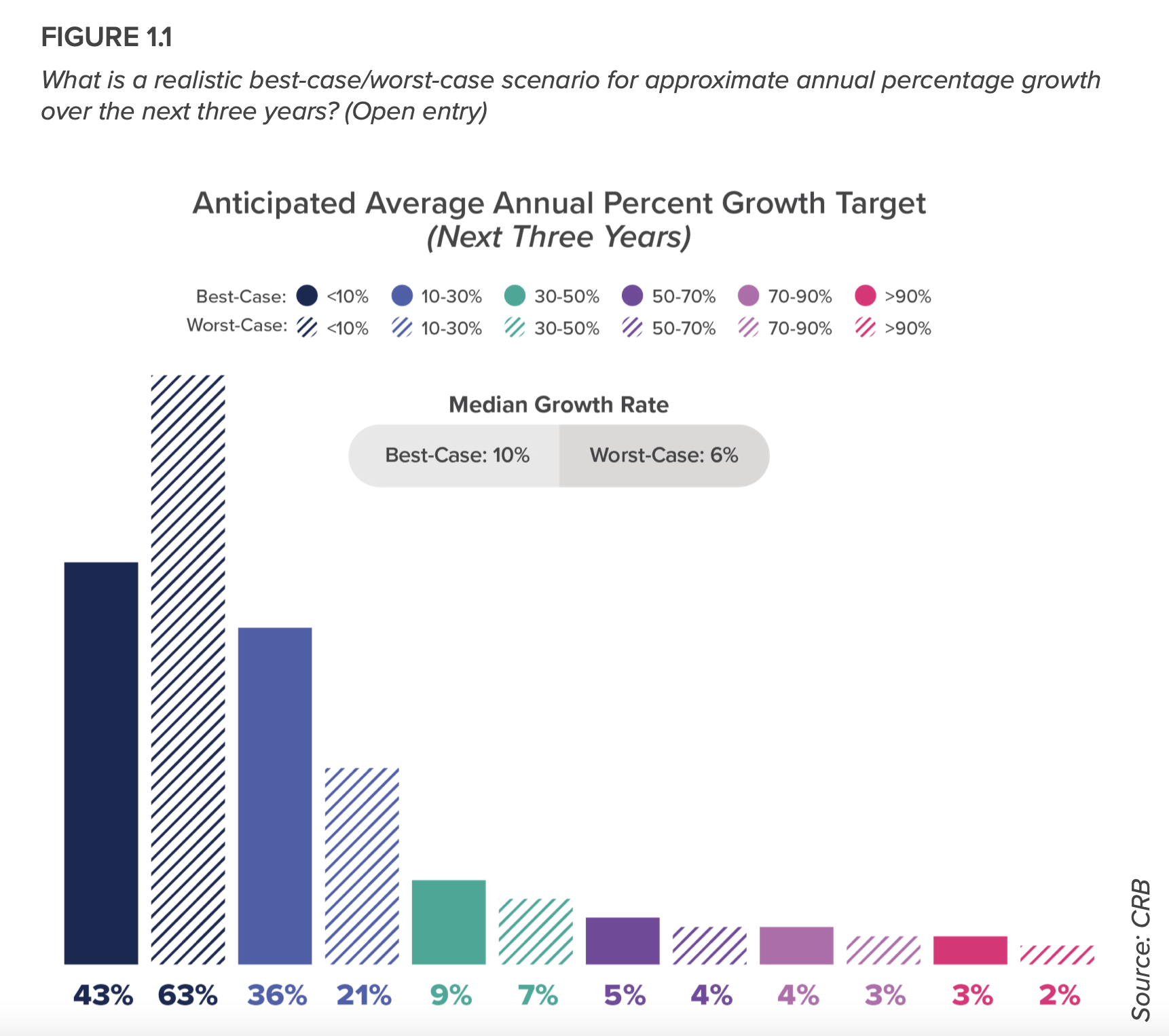
 Cell therapies emerge as dynamic submarket
Cell therapies emerge as dynamic submarket
CRB divides its report into eight chapters, each with contextualized perspectives. Those chapters found that:
- Since the pandemic, companies have adopted an optimistic but more cautious approach to ongoing research and discovery. That means carefully weighing risks and rewards of capital spending and pipeline expansion while continuously pushing for new and exciting discoveries.
- Ribonucleic acid (RNA) technologies have catapulted into the spotlight because of COVID. In addition to preventing infectious diseases, these technologies—using non-coding and coding RNA—can be harnessed to treat other conditions, like cancer. When compared to other biologics, RNA technologies have the potential to increase speed to market, lower costs, and reduce regulatory requirements.
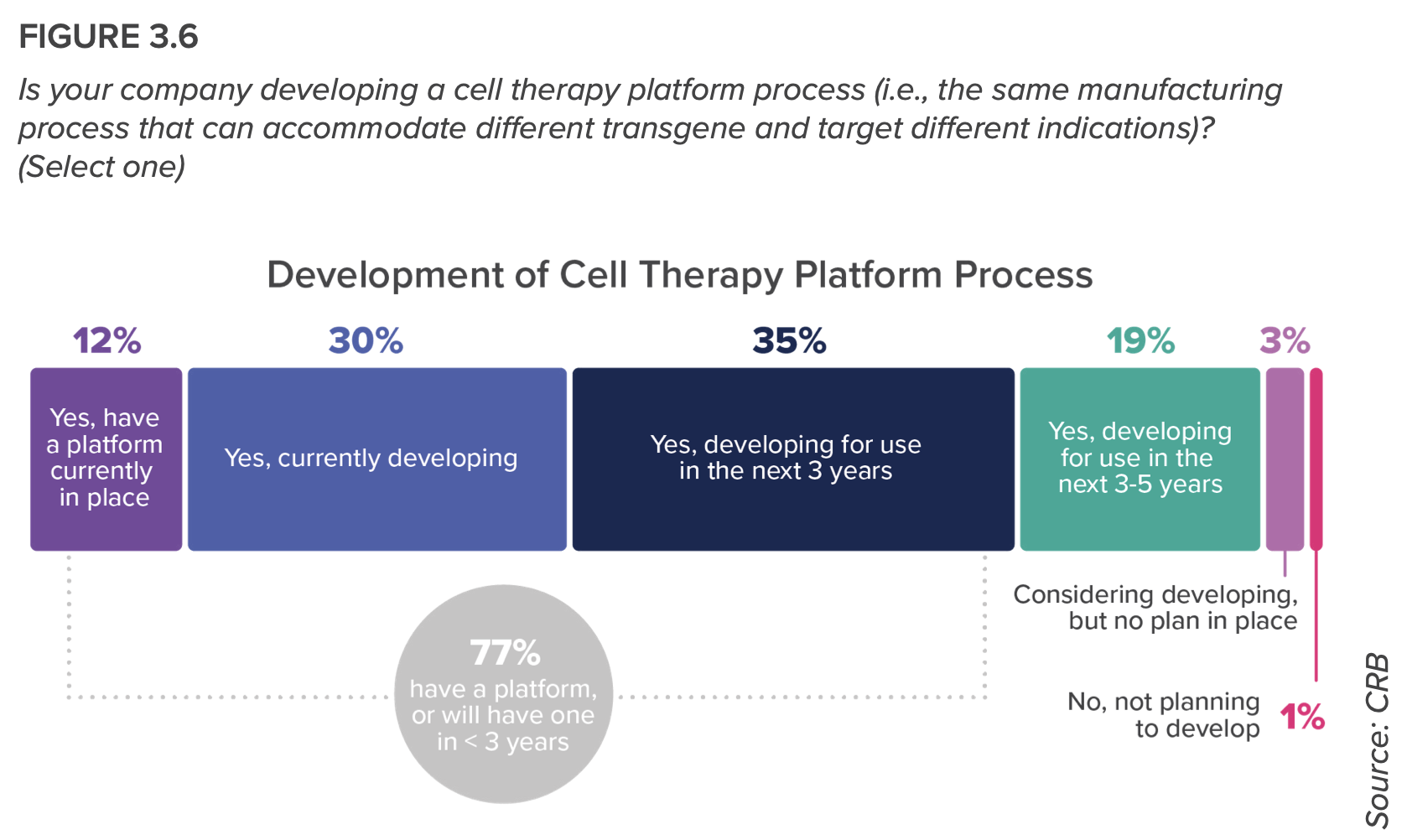
- More than 300 of this report’s respondents have cell therapies in their pipelines, creating one of the most dynamic—and challenging—submarkets. Researchers are leveraging standardized platforms to maximize the versatility and scalability of their processes, to where decentralized manufacturing will change the future of autologous production—“a future that will see cell therapies mature from our last line of defense to an accessible and expected level of patient care,” the report predicts.
- Change is also accelerating for gene therapies. Most respondents said they plan to leap from the small-scale batches necessary for early clinical trials to much larger manufacturing volumes within just three years. Suspension cell cultures, sterile filtration, stable cell lines, and in-house plasmids manufacturing are attracting an enormous volume of R&D activity among both owners and contract manufacturing organizations (CMOs), as this race toward the commercial market heats up.
Modular design will facilitate expansion
- Last year was a milestone year for therapeutic proteins, and not only because the U.S. Food and Drug Administration (FDA) approved the 100th antibody therapy on the market. The field of therapeutic proteins has come a long way—especially in the last few years wherein trends, technologies, and perceptions in the industry saw significant changes. Developers are strategizing for the future.
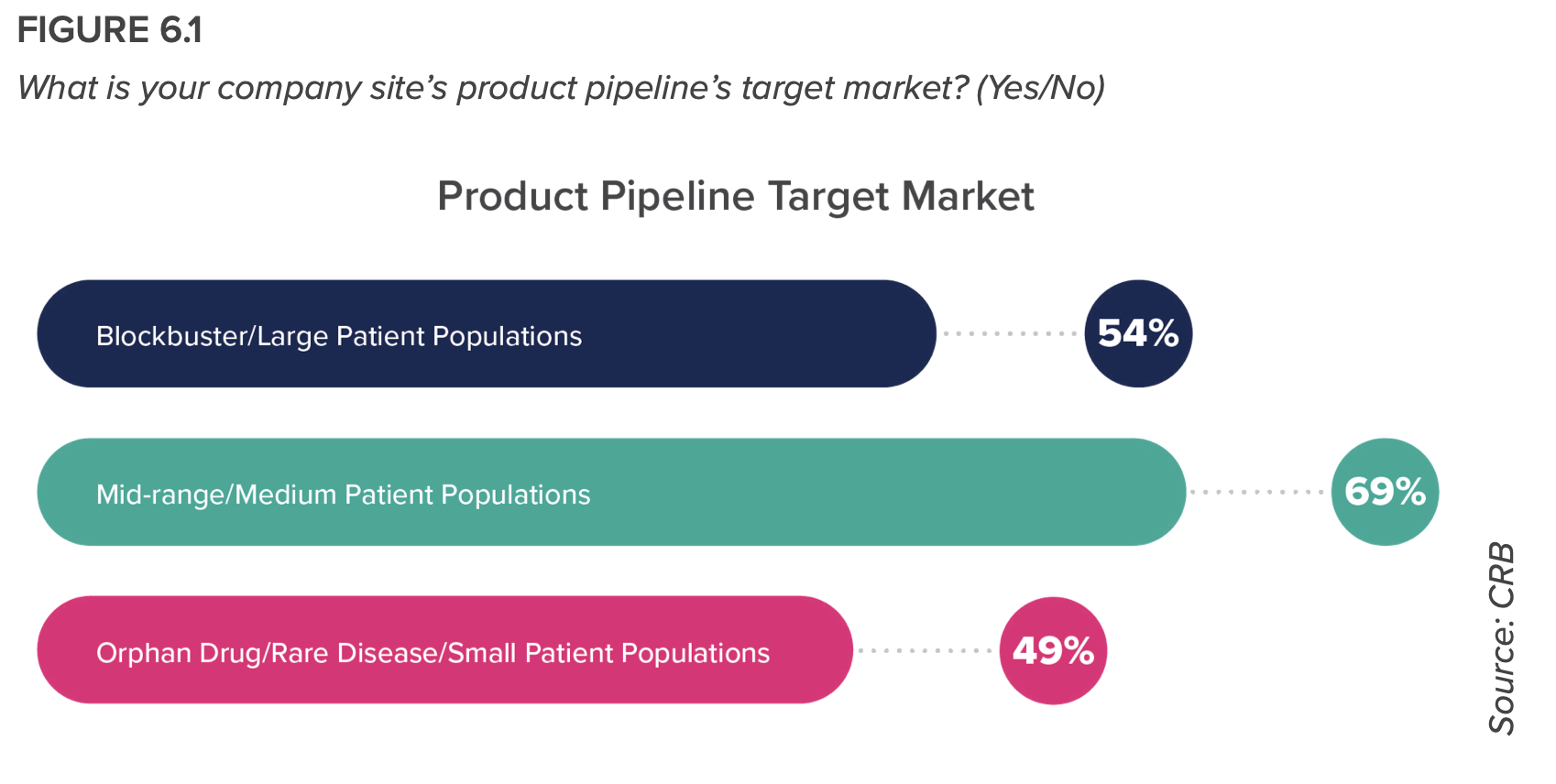
- The tailwinds from COVID-19 treatment innovation have unleashed a new era for drug product manufacturing: one that is looking beyond rare disease markets and smaller patient populations to search for the next blockbuster drug; one that is engaging with drug product formulations that are becoming increasingly more complex; and one that readily embraces automation and online/inline monitoring technologies even at the clinical production operations level.
- CRB’s experts are seeing an “encouraging evolution” in the journey to implementing all aspects of Pharma 4.0—shorthand for efficiencies through process visibility, faster decision making, and real-time system optimization—including smart end-user devices, advanced robotics, and digital twins. “We can see that in the abundance of recent acquisitions that have brought AI innovators into established life science companies,” the report states. But respondents remain sanguine about how to get there, knowing that budget constraints, organizational reluctance, and a lack of skilled labor might hold them back.
- Most respondents plan to expand over the next five years, with some indicating an intent to establish a footprint in other countries. Optimizing capital and operations expenditure in these expansion efforts means standardizing operations between sites, expediting regulatory approvals, and remaining agile to demand for new modalities and technologies. Hence, CRB concludes, a case for modular design.
Related Stories
Laboratories | Nov 8, 2023
Boston’s FORUM building to support cutting-edge life sciences research and development
Global real estate companies Lendlease and Ivanhoé Cambridge recently announced the topping-out of FORUM, a nine-story, 350,000-sf life science building in Boston. Located in Boston Landing, a 15-acre mixed-use community, the $545 million project will achieve operational net zero carbon upon completion in 2024.
Laboratories | Sep 20, 2023
Life sciences construction market is poised for a comeback: JLL
The life sciences commercial real estate market has undergone a reset this year but is well positioned to be the comeback kid as capital sources grow more confident and green shoots emerge, according to JLL’s 2023 Life Sciences Industry and Real Estate Perspective.
Laboratories | Aug 24, 2023
Net-zero carbon science center breaks ground in Canada
Designed by Diamond Schmitt, the new Atlantic Science Enterprise Centre (ASEC) will provide federal scientists and partners with state-of-the-art space and equipment to collaborate on research opportunities.
Giants 400 | Aug 22, 2023
Top 115 Architecture Engineering Firms for 2023
Stantec, HDR, Page, HOK, and Arcadis North America top the rankings of the nation's largest architecture engineering (AE) firms for nonresidential building and multifamily housing work, as reported in Building Design+Construction's 2023 Giants 400 Report.
Giants 400 | Aug 22, 2023
2023 Giants 400 Report: Ranking the nation's largest architecture, engineering, and construction firms
A record 552 AEC firms submitted data for BD+C's 2023 Giants 400 Report. The final report includes 137 rankings across 25 building sectors and specialty categories.
Giants 400 | Aug 22, 2023
Top 175 Architecture Firms for 2023
Gensler, HKS, Perkins&Will, Corgan, and Perkins Eastman top the rankings of the nation's largest architecture firms for nonresidential building and multifamily housing work, as reported in Building Design+Construction's 2023 Giants 400 Report.
University Buildings | Aug 7, 2023
Eight-story Vancouver Community College building dedicated to clean energy, electric vehicle education
The Centre for Clean Energy and Automotive Innovation, to be designed by Stantec, will house classrooms, labs, a library and learning center, an Indigenous gathering space, administrative offices, and multiple collaborative learning spaces.
Laboratories | Jul 10, 2023
U.S. Department of Agriculture opens nation’s first biosafety level 4 containment facility for animal disease research
Replacing a seven-decade-old animal disease center, the National Bio and Agro-Defense Facility includes the nation’s first facility with biosafety containment capable of housing large livestock.
Laboratories | Jun 23, 2023
A New Jersey development represents the state’s largest-ever investment in life sciences and medical education
In New Brunswick, N.J., a life sciences development that’s now underway aims to bring together academics and researchers to work, learn, and experiment under one roof. HELIX Health + Life Science Exchange is an innovation district under development on a four-acre downtown site. At $731 million, HELIX, which will be built in three phases, represents New Jersey’s largest-ever investment in life sciences and medical education, according to a press statement.
University Buildings | May 17, 2023
New UC Irvine health sciences building supports aim to become national model for integrative health
The new College of Health Sciences Building and Nursing & Health Sciences Hall at the University of California Irvine supports the institution’s goal of becoming a national model for integrative health. The new 211,660-sf facility houses nursing, medical doctorate, pharmacy, philosophy, and public health programs in a single building.

















