“While the COVID-19 pandemic taught new lessons about the quick and safe delivery of critical vaccines, the global shock is only now being understood.” That’s the opening observation of the 98-page 2022 Life Sciences Report, published by the AC firm CRB and based on responses from nearly 500 industry leaders, which explores this industry sector’s R&D and growth strategies.
This is CRB’s third Horizons report, and its first to include Europe, where many of the industry’s leading organizations are paving the way through innovation, groundbreaking research, and new and dynamic ways of speeding therapies to patients.
CRB is seeing a rapidly maturing industry that’s in pursuit of more diversification. “The days of single-product specialization are receding,” the report states, as companies large and small are utilizing a wide array of tools to expand their pipeline and address diverse indications.
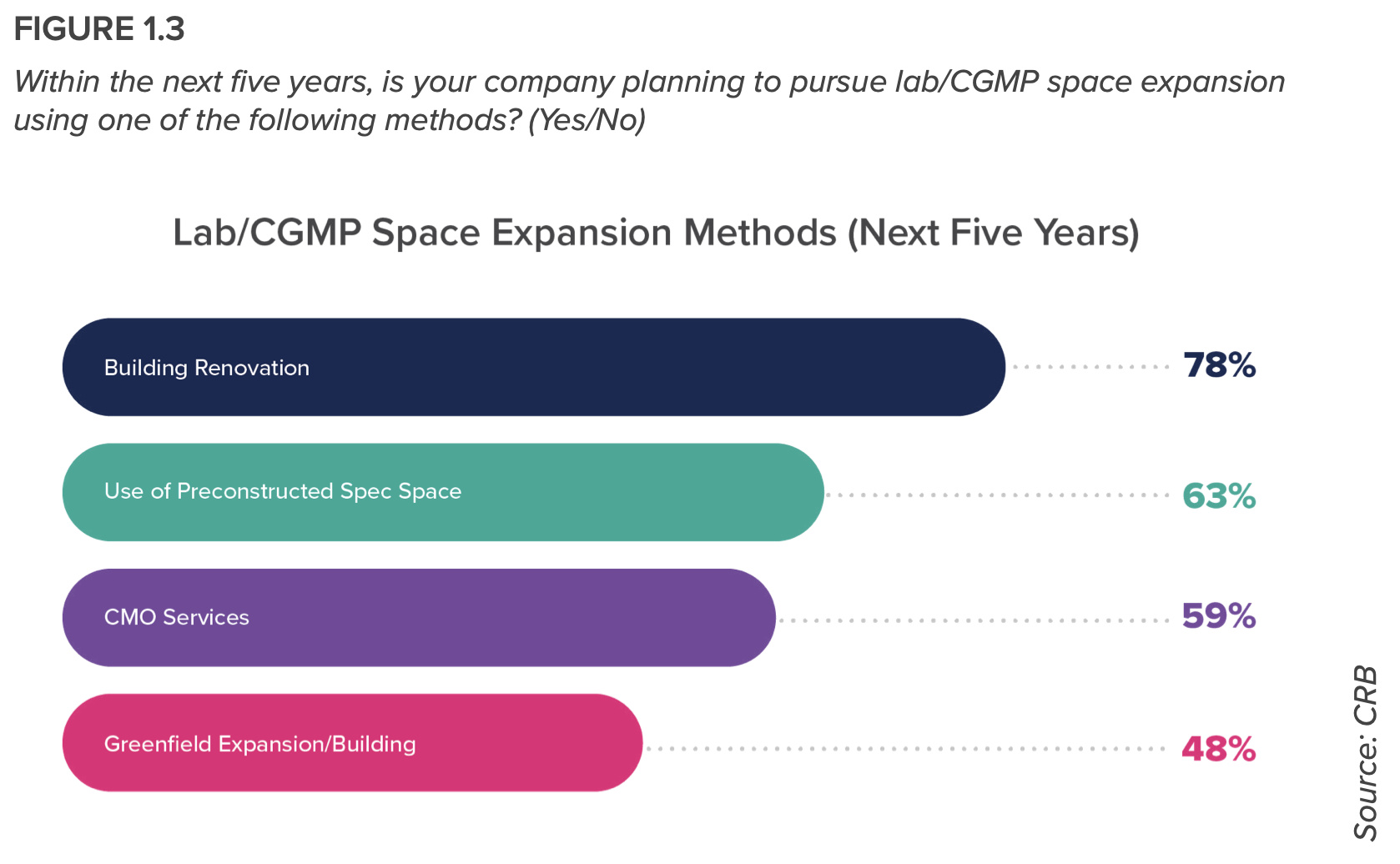
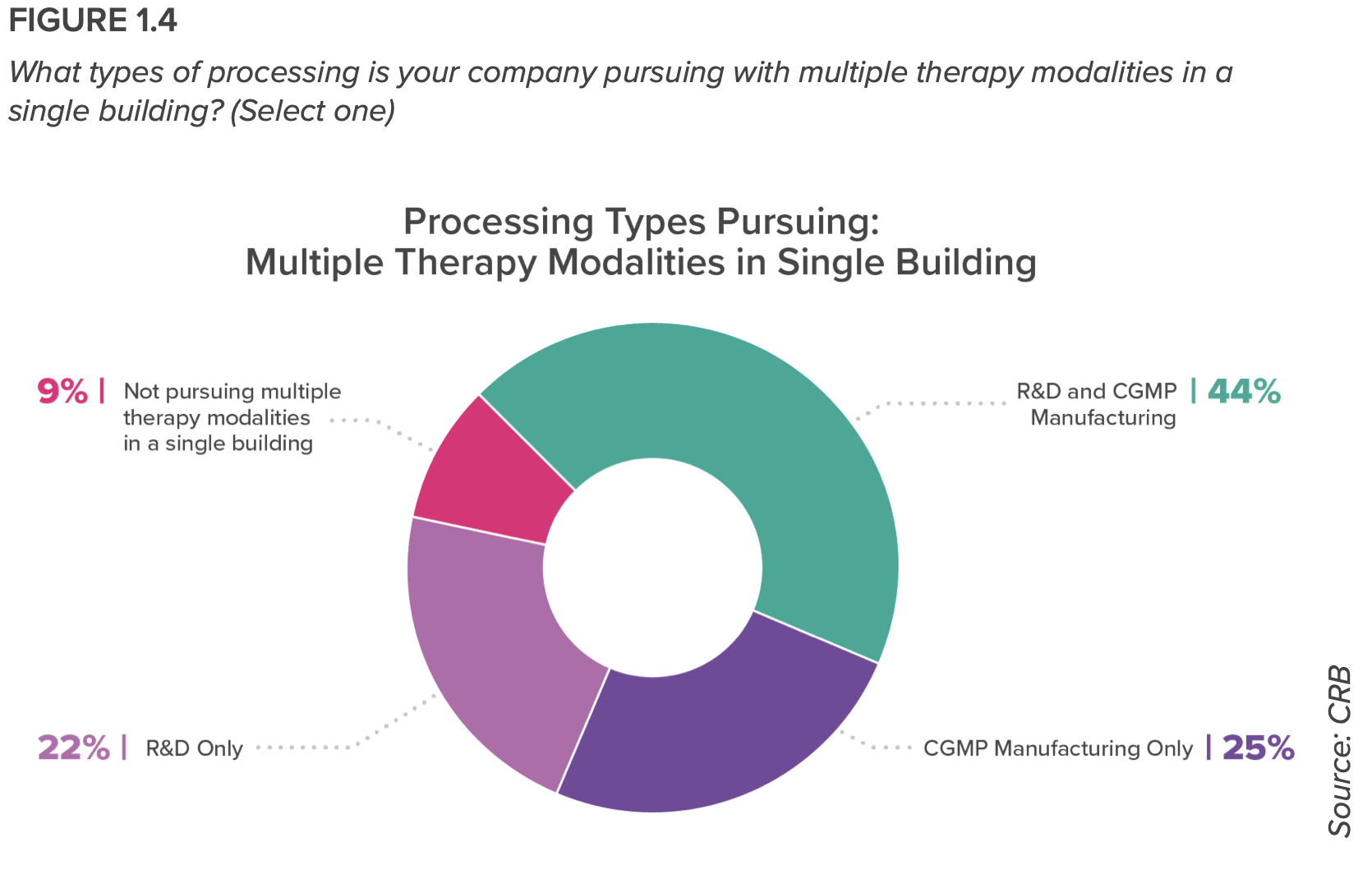
Nearly all respondents (90%) are developing and manufacturing multiple therapy modalities in a single building, or plan to do so in the future. And more than half of the respondents plans to rely on contract development and manufacturing organizations (CDMOs) over the next three years. “We’re seeing a rise in hybrid models—that is, owners who are offering their in-house manufacturing expertise for hire,” the report states.
As such, the nature of in-demand talent is changing, as companies mature towards more automated, AI-driven manufacturing models, with the traditional C-suite expanding to include roles previously unseen in this industry, such as “Chief Data Officer.”
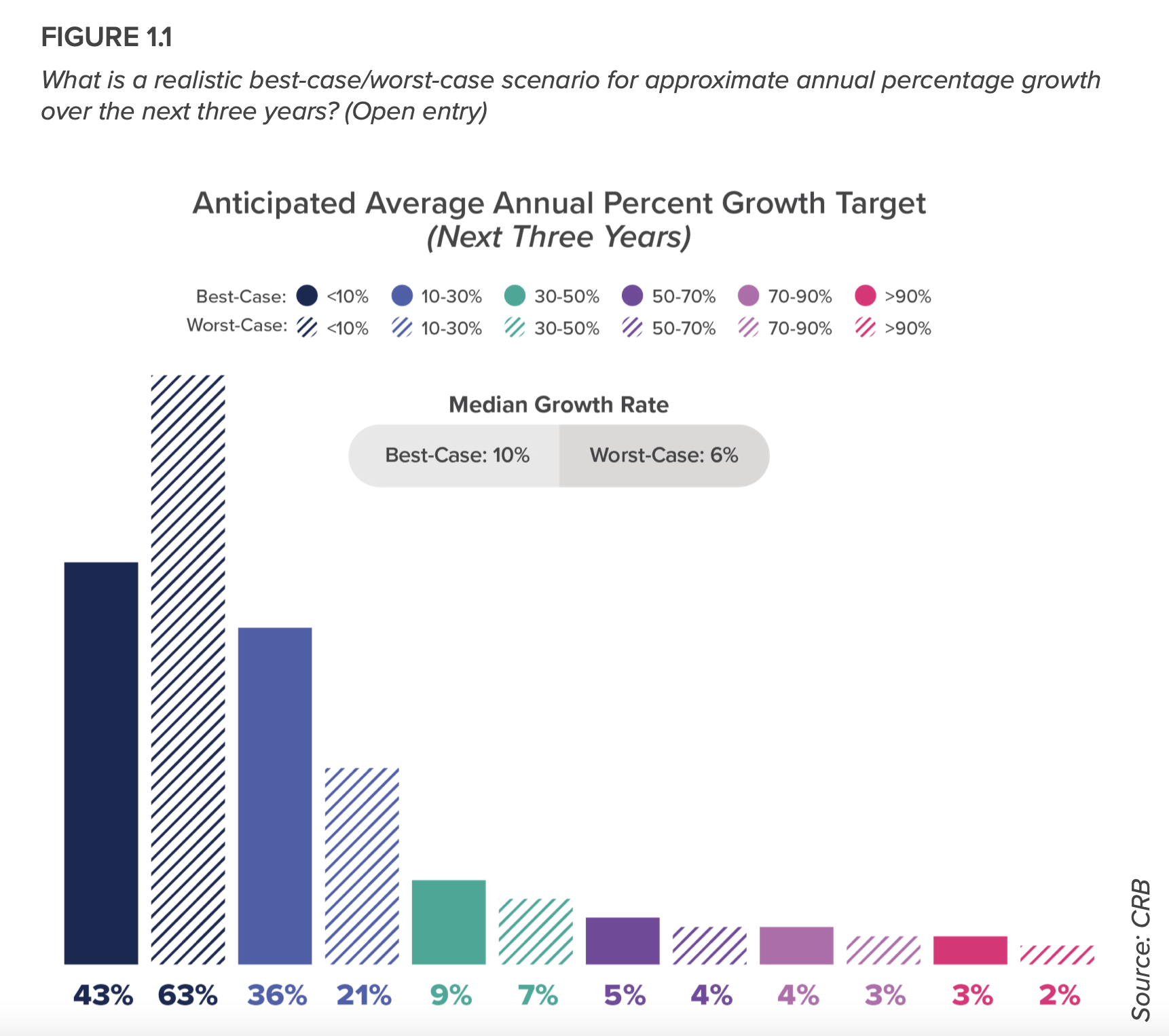
 Cell therapies emerge as dynamic submarket
Cell therapies emerge as dynamic submarket
CRB divides its report into eight chapters, each with contextualized perspectives. Those chapters found that:
- Since the pandemic, companies have adopted an optimistic but more cautious approach to ongoing research and discovery. That means carefully weighing risks and rewards of capital spending and pipeline expansion while continuously pushing for new and exciting discoveries.
- Ribonucleic acid (RNA) technologies have catapulted into the spotlight because of COVID. In addition to preventing infectious diseases, these technologies—using non-coding and coding RNA—can be harnessed to treat other conditions, like cancer. When compared to other biologics, RNA technologies have the potential to increase speed to market, lower costs, and reduce regulatory requirements.
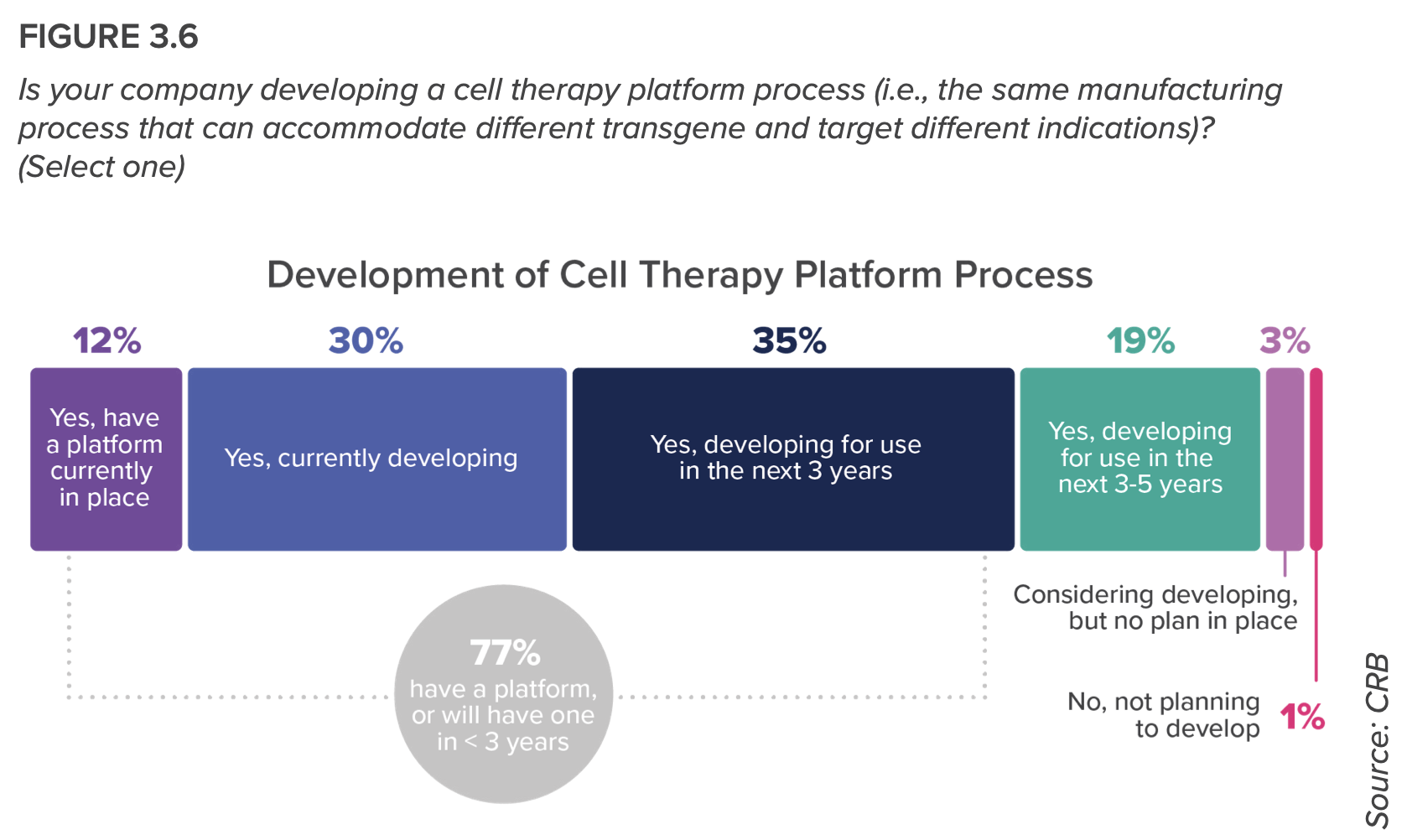
- More than 300 of this report’s respondents have cell therapies in their pipelines, creating one of the most dynamic—and challenging—submarkets. Researchers are leveraging standardized platforms to maximize the versatility and scalability of their processes, to where decentralized manufacturing will change the future of autologous production—“a future that will see cell therapies mature from our last line of defense to an accessible and expected level of patient care,” the report predicts.
- Change is also accelerating for gene therapies. Most respondents said they plan to leap from the small-scale batches necessary for early clinical trials to much larger manufacturing volumes within just three years. Suspension cell cultures, sterile filtration, stable cell lines, and in-house plasmids manufacturing are attracting an enormous volume of R&D activity among both owners and contract manufacturing organizations (CMOs), as this race toward the commercial market heats up.
Modular design will facilitate expansion
- Last year was a milestone year for therapeutic proteins, and not only because the U.S. Food and Drug Administration (FDA) approved the 100th antibody therapy on the market. The field of therapeutic proteins has come a long way—especially in the last few years wherein trends, technologies, and perceptions in the industry saw significant changes. Developers are strategizing for the future.
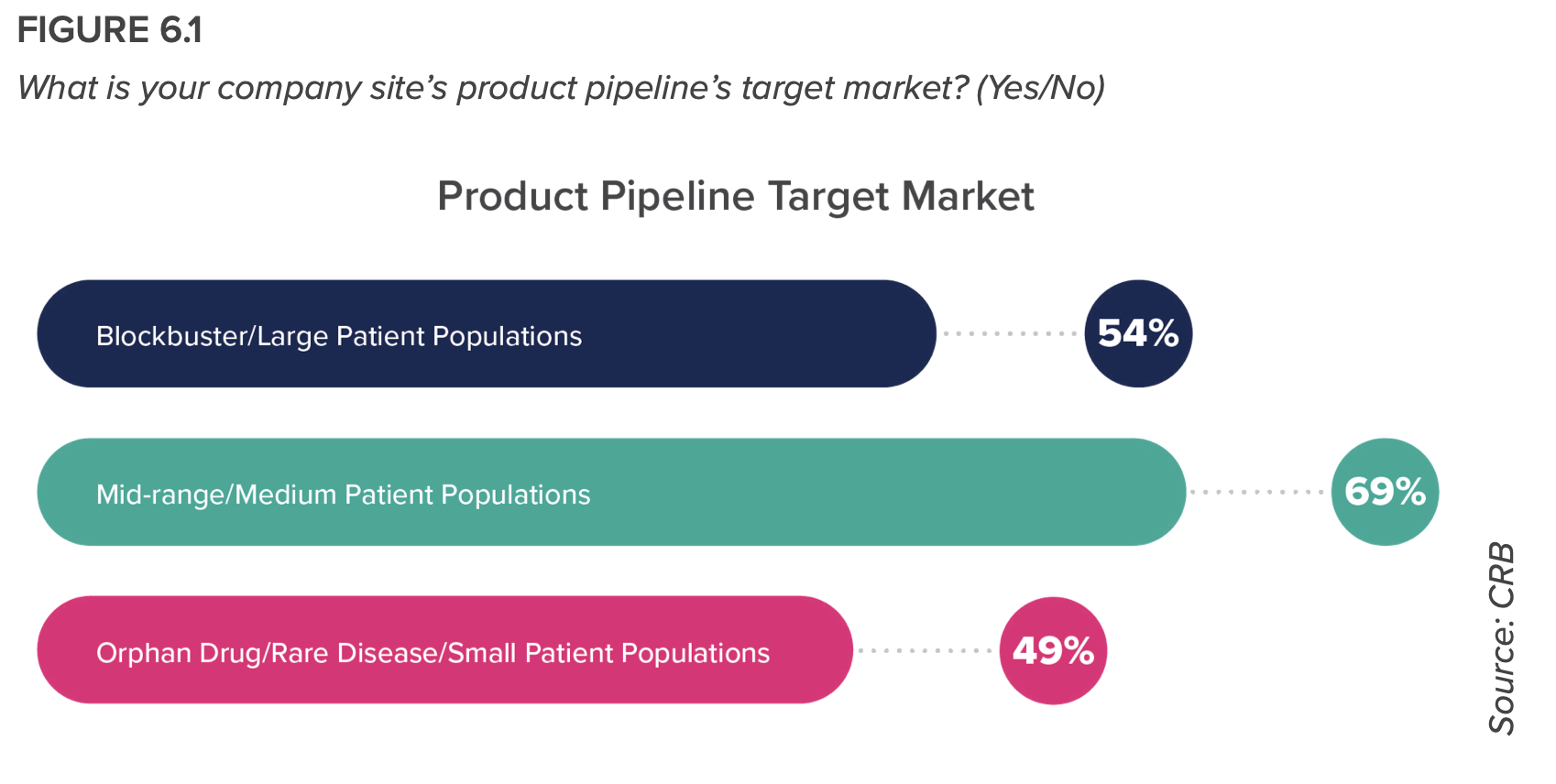
- The tailwinds from COVID-19 treatment innovation have unleashed a new era for drug product manufacturing: one that is looking beyond rare disease markets and smaller patient populations to search for the next blockbuster drug; one that is engaging with drug product formulations that are becoming increasingly more complex; and one that readily embraces automation and online/inline monitoring technologies even at the clinical production operations level.
- CRB’s experts are seeing an “encouraging evolution” in the journey to implementing all aspects of Pharma 4.0—shorthand for efficiencies through process visibility, faster decision making, and real-time system optimization—including smart end-user devices, advanced robotics, and digital twins. “We can see that in the abundance of recent acquisitions that have brought AI innovators into established life science companies,” the report states. But respondents remain sanguine about how to get there, knowing that budget constraints, organizational reluctance, and a lack of skilled labor might hold them back.
- Most respondents plan to expand over the next five years, with some indicating an intent to establish a footprint in other countries. Optimizing capital and operations expenditure in these expansion efforts means standardizing operations between sites, expediting regulatory approvals, and remaining agile to demand for new modalities and technologies. Hence, CRB concludes, a case for modular design.
Related Stories
Architects | Aug 5, 2021
Lord Aeck Sargent's post-Katerra future, with LAS President Joe Greco
After three years under the ownership of Katerra, which closed its North American operations last May, the architecture firm Lord Aeck Sargent is re-establishing itself as an independent company, with an eye toward strengthening its eight practices and regional presence in the U.S.
Laboratories | May 6, 2021
The big shift: How laboratory design should respond to personalized medicine
Crucial to the success of personalized medicine is the “big shift” away from large-scale pharmaceutical manufacturing to small-scale lab manufacturing.
Laboratories | Mar 10, 2021
8 tips for converting office space to life sciences labs
Creating a successful life sciences facility within the shell of a former office building can be much like that old “square peg round hole” paradigm. Two experts offer important advice.
Giants 400 | Dec 3, 2020
2020 Science & Technology Facilities Giants: Top architecture, engineering, and construction firms in the S+T sector
HDR, Jacobs, and Turner head BD+C's rankings of the nation's largest science and technology (S+T) facilities sector architecture, engineering, and construction firms, as reported in the 2020 Giants 400 Report.
Giants 400 | Dec 3, 2020
2020 Laboratory Facilities Sector Giants: Top architecture, engineering, and construction firms in the U.S. laboratory facilities sector
Affiliated Engineers, HDR, and Skanska top BD+C's rankings of the nation's largest laboratory facilities sector architecture, engineering, and construction firms, as reported in the 2020 Giants 400 Report.
Laboratories | Nov 16, 2020
Washington State University’s new Plant Sciences Building opens
LMN Architects designed the project.
AEC Tech Innovation | Sep 18, 2020
New Innovation Center should heighten Port San Antonio’s tech profile
The facility will include a 2,500-seat arena and serve as new home for the city’s S&T museum.
Laboratories | Aug 25, 2020
Video: What's driving the boom in life sciences real estate?
JLL's Audrey Symes discusses the drivers of growth across the nation's life sciences cluster hubs.
Laboratories | Jul 24, 2020
Customized labs give universities a recruiting edge
CO Architects is among a handful of firms that caters to this trend.
















